Novel fluorescence imaging techniques
Fluorescence imaging, characterized by high sensitivity and specificity, is one of the driving horse in modern biological research.
However, when imaging highly scattering biological tissues (such as the brain) using wide-field fluorescence, the resolution significantly decreases,
and there is a lack of depth information. Although scanning fluorescence imaging methods (such as confocal and multiphoton imaging) can reduce
the effects of scattering and obtain depth information, they are often limited by small imaging fields and slow scanning speeds.
To address these bottlenecks, novel fluorescence imaging techniques are desired.
So far, the following imaging method has been proposed within the group.
(1) High-speed scanning large-field fluorescence imaging technology based on multifocal illumination
To achieve high spatial resolution and high-speed imaging over a large field, a large-field multifocal illumination (LMI) scanning microscopy method
based on an acousto-optic deflector (AOD) and a diffraction grating has been proposed (Chen and McLarney et al., Laser & Photonics Reviews, 2020)
(Figure 1). Typically, while AODs can achieve high-speed beam scanning, they are limited by a small scanning angle (< 3°), making large-field imaging
unfeasible. However, diffraction gratings can generate multiple orders of diffracted light, significantly compressing the scanning angle.
By cleverly combining the advantages of these two optical components, ultra-fast, ultra-large field high-resolution imaging can be achieved.
This method serves as a universal technology that breaks the bottleneck of traditional scanning microscopy and is also applicable to other scanning
imaging systems (Chen and Özbek et al., Light Science & Applications, 2020).
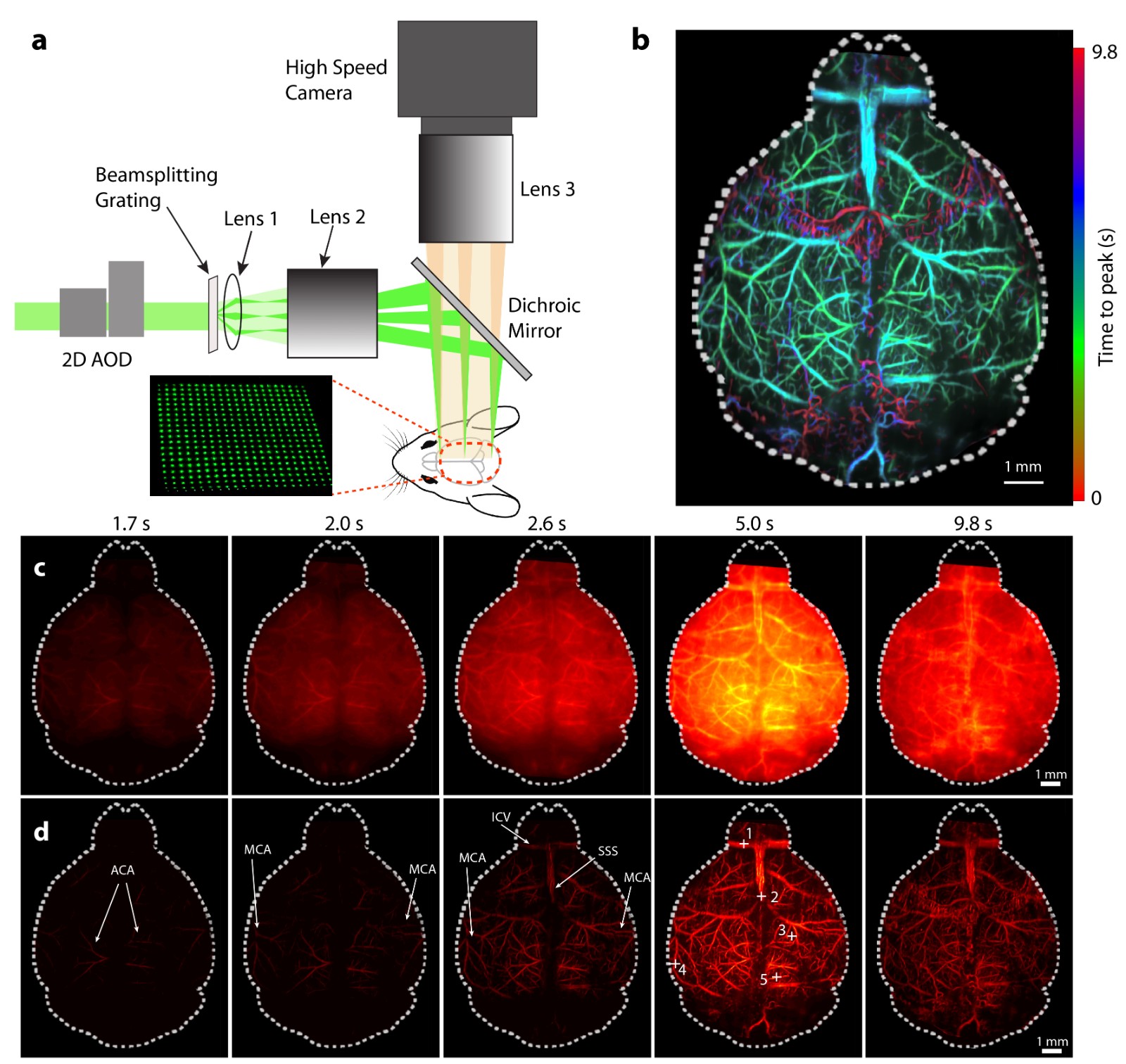
Fig. 1 Large field multifocal illumination fluorescence microscopy and its application in imaging of mouse brain perfusion dynamics.
(2) Three-dimensional large-field high-speed fluorescence imaging technology based on astigmatic depth encoding
To address the limitation of traditional wide-field fluorescence microscopy in obtaining spatial depth information while maintaining the advantages of
large-field high-speed imaging, a large-field multifocal three-dimensional (3D-LMI) fluorescence microscopy method based on optical astigmatic depth
encoding has been proposed (Zhou* and Chen* et al., Nature Communications, 2022). Similar to the LMI method, the 3D-LMI method projects a
"lattice" structured light pattern onto the surface of the sample to be tested, utilizing a customized cylindrical lens to implement point spread function
(PSF) depth encoding. Combined with post-processing image analysis, this approach effectively encodes a depth range of approximately 600 μm
(Figure 2). This method represents the first exploration of ultra-large field three-dimensional fluorescence imaging, applied in studies of cell tracking
within cortical tissue and the activity of neurons across the entire brain cortex.
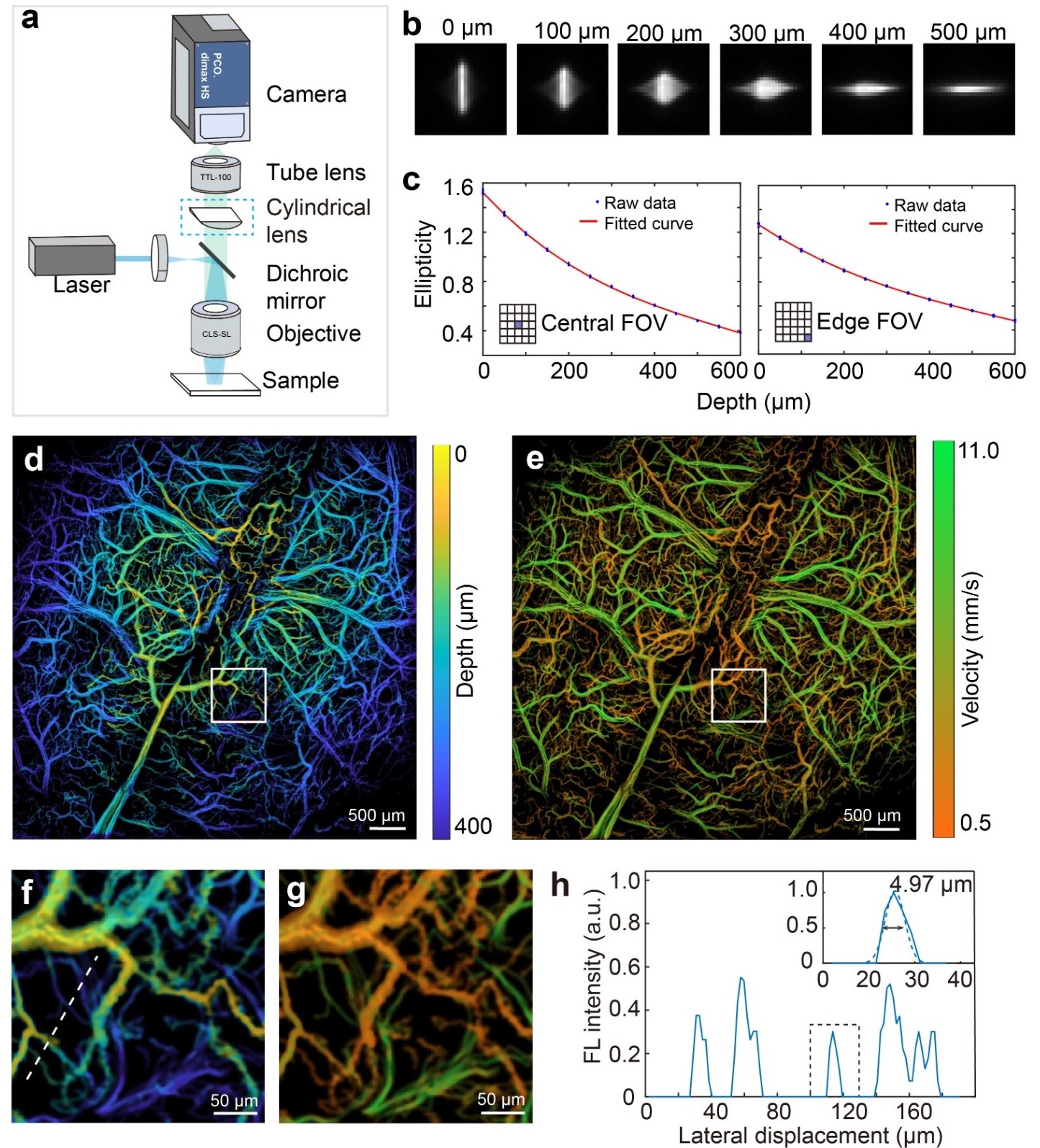
Fig. 2 Three-dimensional large field multifocal illumination fluorescence microscopy.
(3) Wide-field Fluorescence Localization Microscopy
Due to the strong scattering of light signals in living tissues, traditional fluorescence microscopy methods require long scanning times under cranial
windows to obtain high-resolution images of the cerebral vascular network. To quickly acquire real-time measurements of blood flow velocity and
direction at capillary resolution across the entire brain with minimal invasiveness, a wide-field fluorescence localization microscopy method has been
proposed (Chen et al., Optics Letters, 2020). This method is based on the localization and tracking of sparsely distributed fluorescent particles in the
blood, allowing for quantitative measurements of transcranial blood flow velocity and direction at a capillary-level resolution (5 µm), providing an
important avenue for studying cerebral circulation and brain function non-invasively. However, functional brain imaging imposes higher requirements
on fluorescent markers: 1) they must have a long circulation time in vivo; 2) they must accurately reflect changes in blood flow. To address these
requirements, a functional brain imaging method based on fluorescently labeled red blood cells has been further proposed (Figure 3)
(Zhou et al., Chen, Nature Communications, 2020). By simultaneously capturing changes in neuronal fluorescence calcium signals, this method
reveals the coupling relationship between neuronal activity across the entire cortical scale of mice and changes in cerebral blood flow, providing
a powerful tool for studying neurovascular coupling, neuro-metabolic mechanisms, and the pathology of neurodegenerative diseases (Figure 4).
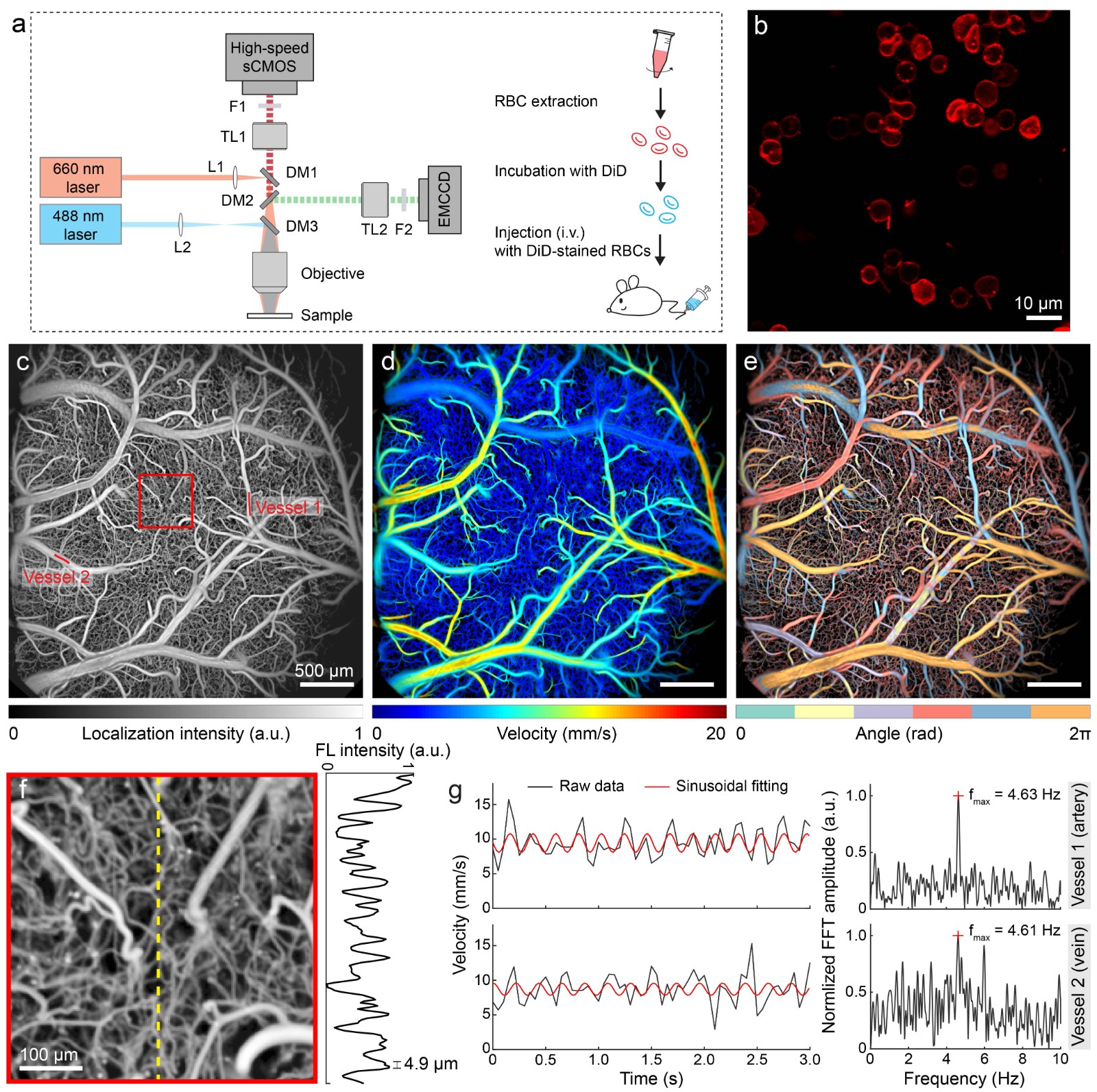
Fig. 3 Whole-cortex high-resolution functionaling neuroimaging with fluorescently labeled RBCs.
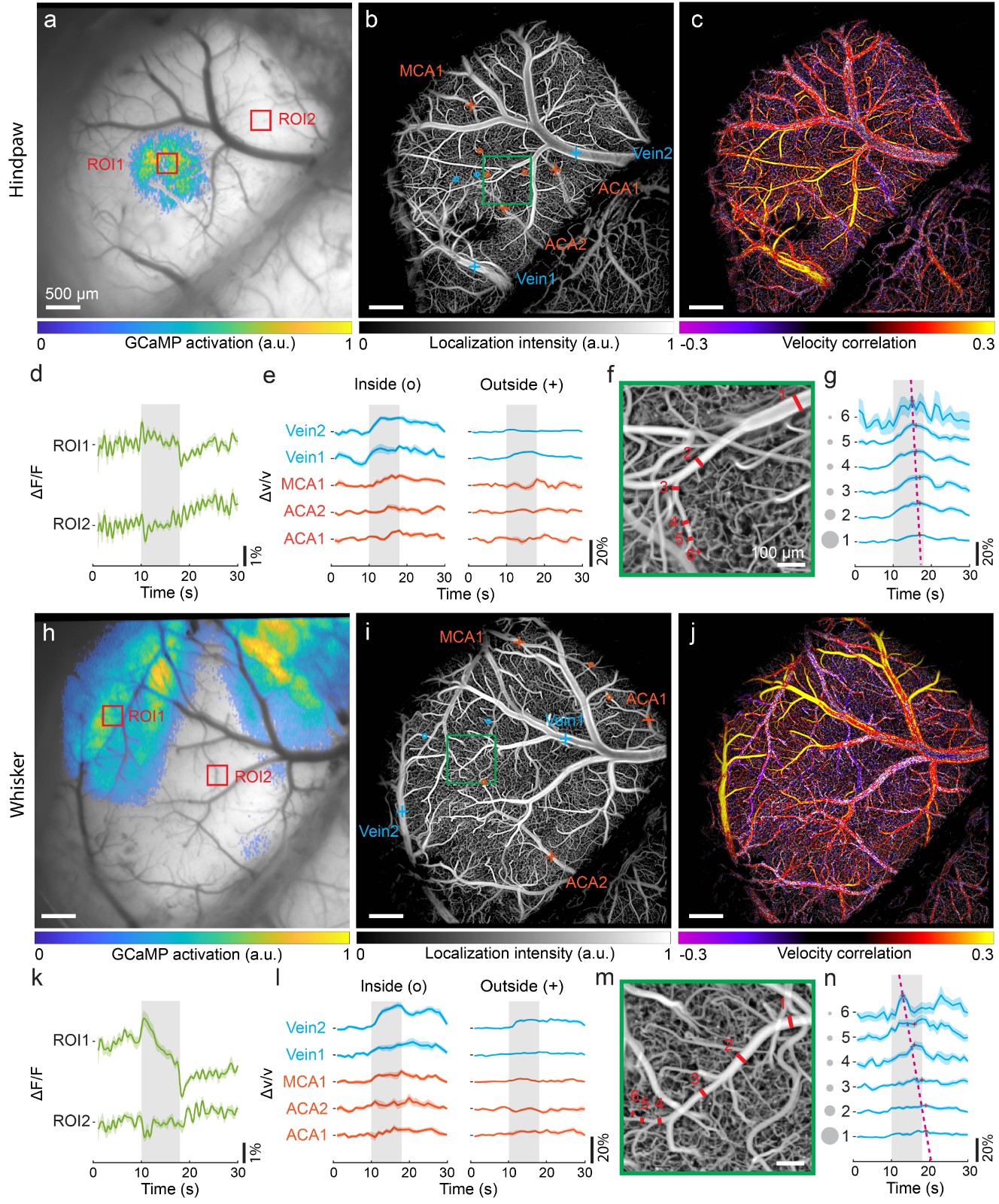
Fig. 4 Co-activation patterns of neuronal calcium signal and induced hemodynamic responses with electrical hindpaw/whisker stimulation paradigm.
References
Z. Chen et al., Transcranial cortex-wide imaging of murine ischemic perfusion with large-field multifocal illumination fluorescence microscopy. 10.1101/2023.11.01.564959 %J bioRxiv, 2023.2011.2001.564959 (2023).
Q. Zhou et al., Three-dimensional wide-field fluorescence microscopy for transcranial mapping of cortical microcirculation. 13, 7969 (2022).
R. Ni, Z. Chen, X. L. Deán-Ben, F. F. Voigt, G. S. Daniel Kirschenbaum, Alessia Villois, Quanyu Zhou, Alessandro Crimi, Paolo Arosio, Roger M. Nitsch, K. Peter R. Nilsson, Adriano Aguzzi, Fritjof Helmchen, Jan Klohs, Daniel Razansky, Multiscale optical and optoacoustic imaging of amyloid-β deposits in mice. Nature Biomedical Engineering, (2022).
Q. Zhou, Z. Chen, J. Robin, X.-L. Deán-Ben, D. J. O. Razansky, Diffuse optical localization imaging for noninvasive deep brain microangiography in the NIR-II window. 8, 796-803 (2021).
Z. Chen, Q. Zhou, J. Robin, D. Razansky, Widefield fluorescence localization microscopy for transcranial imaging of cortical perfusion with capillary resolution. Optics letters 45, 3470-3473 (2020).
Z. Chen, Q. Zhou, J. Rebling, D. Razansky, Cortex‐wide microcirculation mapping with ultrafast large‐field multifocal illumination microscopy. Journal of biophotonics 13, e202000198 (2020).
Z. Chen et al., High‐Speed Large‐Field Multifocal Illumination Fluorescence Microscopy. Laser & photonics reviews 14, 1900070 (2020).
Q. Zhou et al., Cortex-wide transcranial localization microscopy with fluorescently labeled red blood cells. Nature Communications 15, 3526 (2024).
C. Glück et al., Pia-FLOW: Deciphering hemodynamic maps of the pial vascular connectome and its response to arterial occlusion. Proc Natl Acad Sci U S A 121, e2402624121 (2024).




